Ethylene-vinyl acetate
This article needs additional citations for verification. (December 2015) |
| |
| Names | |
|---|---|
| Other names
Poly(ethylene-vinyl acetate); poly(ethylene-co-vinyl acetate); polyethylene-vinyl acetate copolymer
| |
| Identifiers | |
| Abbreviations | EVA; PEVA |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.133.085 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
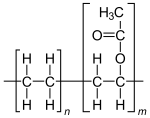
| (C2H4)n(C4H6O2)m | |
| Molar mass | Variable |
| Melting point | 90 °C (194 °F) |
| Hazards | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |


Ethylene-vinyl acetate (EVA), also known as poly(ethylene-vinyl acetate) (PEVA), is a copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 50%, with the remainder being ethylene. There are three different types of EVA copolymer, which differ in the vinyl acetate (VA) content and the way the materials are used.
The EVA copolymer which is based on a low proportion of VA (approximately up to 4%) may be referred to as vinyl acetate modified polyethylene. It is a copolymer and is processed as a thermoplastic material – just like low-density polyethylene. It has some of the properties of a low-density polyethylene but increased gloss (useful for film), softness and flexibility. The material is generally considered non-toxic.
The EVA copolymer which is based on a medium proportion of VA (approximately 4 to 30%) is referred to as thermoplastic ethylene-vinyl acetate copolymer and is a thermoplastic elastomer material. It is not vulcanized but has some of the properties of a rubber or of plasticized polyvinyl chloride particularly at the higher end of the range. Both filled and unfilled EVA materials have good low temperature properties and are tough. The materials with approximately 11% VA are used as hot-melt adhesives.
The EVA copolymer which is based on a high proportion of VA (greater than 60%) is referred to as ethylene-vinyl acetate rubber.[1]
EVA is an elastomeric polymer that produces materials which are "rubber-like" in softness and flexibility. The material has good clarity and gloss, low-temperature toughness, stress-crack resistance, hot-melt adhesive waterproof properties, and resistance to UV radiation. EVA has a distinctive vinegar-like odor and is competitive with rubber and vinyl polymer products in many electrical applications.
Production
[edit]This section needs expansion. You can help by adding to it. (October 2022) |
EVA is made by mixing ethylene and vinyl acetate in a processor, which creates an unrefined mass of EVA. It is fed through rollers that flatten it into sheets, which are then put into a pressure oven.[2] Ethylene-vinyl acetate is based on products from the production of petroleum and natural gas.
Hydrolysis of EVA gives ethylene vinyl alcohol (EVOH) copolymer (and acetic acid).
Applications
[edit]- Some objects made of EVA
-
Foam flutterboard
-
EVA foam sheets
-
Garden kneeler
-
Flip-flop sole
-
Foam Crocs
-
Yoga blocks are commonly made of EVA, as are some mats
-
Interlocking floor tiles are often made from EVA foam
Hot-melt adhesives (such as hot glue sticks) and top-of-the-line soccer cleats are usually made from EVA, generally with additives like wax and resin. EVA is also used as a clinginess-enhancing additive in plastic wraps. Craft-foam sheets are made of EVA and are popularly used for children's foam stickers.[clarification needed]
EVA is also used in biomedical engineering applications as a drug-delivery device. The polymer is dissolved in an organic solvent (such as dichloromethane). Powdered drug and filler (typically an inert sugar) are added to the liquid solution and rapidly mixed to obtain a homogeneous mixture. The drug-filler-polymer mixture is then cast into a mold at −80 °C and freeze-dried until solid. These devices are used in drug delivery research to slowly release a compound. The polymer does not biodegrade within the body, but is quite inert and causes little or no reaction following implantation.
EVA is one of the materials popularly known as expanded rubber or foam rubber. EVA foam[3][4][5] is used as padding in equipment for various sports such as ski boots, bicycle saddles, hockey pads, boxing and mixed-martial-arts gloves and helmets, wakeboard boots, waterski boots, fishing rods, and fishing-reel handles. It is typically used as a shock absorber in sports shoes, for example. (Some manufacturers of running shoes, such as Nike, market EVA-based compression-moulded foam used in the manufacture of running shoes as "Phylon".[6]) It is used for the manufacture of floats for commercial fishing gear such as purse seine (seine fishing) and gillnets. In addition, because of its buoyancy, EVA has made its way into non-traditional products such as floating eyewear. It is also used in the photovoltaics industry as an encapsulation material for crystalline silicon solar cells in the manufacture of photovoltaic modules. EVA slippers and sandals are popular, being lightweight, easy to form, odourless, glossy, and cheaper than natural rubber. In fishing rods, EVA is used to construct handles on the rod-butt end. EVA can be used as a substitute for cork in many applications.
EVA copolymers are adhesives used in packaging, textile, bookbinding for bonding plastic films, metal surfaces, coated paper, and as redispersible powders in plasters and cement renders.
In recent years, EVA foam has seen popular use in cosplay communities, largely in part due to its ease to work with, durability, and comfort in comparison to traditional plastic-based costumes.[7]
Flower-making foam is a thin sheet made of EVA, which is flexible, and is used by artists and craft makers to make artificial flowers. These foams are presented as raw sheets and they can be cut into the desired petal shape and then can be formed by ironing to assemble artificial flowers by putting these petals together.[8]
EVA is also used in coatings formulation of good-quality interior water-borne paints at 53% primary dispersant.
Other uses
[edit]EVA is used in orthotics, surfboard and skimboard traction pads, car mats, and for the manufacturing of some artificial flowers.
EVA is used as a cold flow improver for diesel fuel. They are added at the 50-250 ppm level to diesel fuels to inhibit crystallization of waxes which could block fuel filters.[9]
EVA is a separator in HEPA filters. EVA can easily be cut from sheets and molded to shape. It is also used to make thermoplastic mouthguards that soften in boiling water for a user-specific fit. It is also used for conditioning and waterproofing fabrics and leather.[10] EVA finds application in the making of nicotine transdermal patches, since the copolymer binds well with other agents to form gel-like substances. EVA is also sometimes used as a material for some plastic model kit parts. One common use of EVA foam rubber is in low frequency (woofer) speaker cone membrane support rings[11] (replacing rubber) because of its good mechanical and acoustic properties. Open cell EVA foam is used to damp high frequency acoustical diffraction from tweeter speakers and is often put in the area around the high frequency speaker driver to give better directivity and sonic imaging.
EVA may be used in custom-made dental devices with a proper approach to hygiene.[12]
Safety and environmental considerations
[edit]Polyethylene vinyl acetate has recently become a popular alternative to polyvinyl chloride because it does not contain chlorine.[13] As of 2014, EVA has not been found to be carcinogenic by the NTP, ACGIH, IARC, or OSHA, and has no known adverse effect on human health.[14] Like many plastics, it is difficult to biodegrade. One study suggested it may have adverse effects on certain organisms, but its actual effect on humans has not been determined.[13]
See also
[edit]References
[edit]- ^ Whelan, A.; Whelan, Tony (27 May 1994). Polymer Technology Dictionary. Springer Science & Business Media. ISBN 9780412581809 – via Google Books.
- ^ "FindSourcing – EVA - Ethylene-vinly Acetate". www.findsourcing.com. Retrieved 2023-02-15.
- ^ Reyes-Labarta, J.A.; Marcilla, A. (2012). "Thermal Treatment and Degradation of Crosslinked Ethylene Vinyl Acetate-Polyethylene-Azodicarbonamide-ZnO Foams. Complete Kinetic Modelling and Analysis". Industrial & Engineering Chemistry Research. 51 (28): 9515–9530. doi:10.1021/ie3006935.
- ^ Reyes-Labarta, J.A.; Marcilla, A. (2008). "Differential Scanning Calorimetry Analysis of the Thermal Treatment of Ternary Mixtures of Ethylene Vinyl Acetate, Polyethylene and Azodicarbonamide". Journal of Applied Polymer Science. 110 (5): 3217–3224. doi:10.1002/app.28802. hdl:10045/13312.
- ^ Reyes-Labarta, J.A.; Olaya, M.M.; Marcilla, A. (2006). "DSC Study of the Transitions Involved in the Thermal Treatment of Foamable Mixtures of PE and EVA Copolymer with Azodicarbonamide". Journal of Applied Polymer Science. 102 (3): 2015–2025. doi:10.1002/app.23969. hdl:10045/24680.
- ^ Mills, N. J. (2003). "Running shoe materials". In Jenkins, Mike (ed.). Materials in Sports Equipment. Vol. 1. Cambridge: Woodhead Publishing. pp. 74, 76. ISBN 9781855738546. Retrieved 2018-11-11.
- ^ Birger, Candace (July 9, 2020). "Cosplay: How to texture EVA foam to upgrade your cosplayer game". Popverse. Retrieved April 24, 2024.
- ^ "What is Flower Making Foam (aka Foamiran)?". Foamiran. October 12, 2017. Retrieved 22 January 2018.
- ^ Werner Dabelstein, Arno Reglitzky, Andrea Schütze and Klaus Reders "Automotive Fuels" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_719.pub2
- ^ "How Nikwax Works". nikwax.com.
- ^ "Subwoofers and woofers construction".
- ^ Ogawa, Taiji; Yamasaki, Sayaka; Honda, Mariko; Terao, Yutaka; Kawabata, Shigetada; Maeda, Yoshinobu (April 2012). "Long-term survival of salivary streptococci on dental devices made of ethylene vinyl acetate". International Journal of Oral Science. 4 (1): 14–18. doi:10.1038/ijos.2012.13. ISSN 2049-3169. PMC 3412658. PMID 22422084.
- ^ a b Meng, Tingzhu Teresa (2014). "Volatile organic compounds of polyethylene vinyl acetate plastic are toxic to living organisms". The Journal of Toxicological Sciences. 39 (5): 795–802. doi:10.2131/jts.39.795. PMID 25242410.
- ^ "Safety Data Sheet (SDS) SDS-101 – Ethylene-Vinyl-Acetate" (PDF). February 10, 2014. Archived from the original (PDF) on 27 April 2021. Retrieved 18 April 2020.
External links
[edit]- List of EVA tradenames (2007; last update 2008; last archived 2022)









